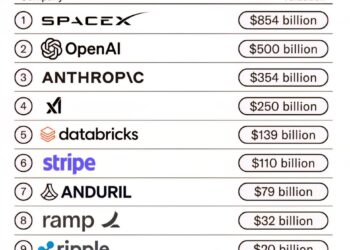
Select Language:
The innovative Chinese pharmaceutical firm experienced a sharp decline today after withdrawing its application for a potentially transformative hepatitis B treatment. The company’s stock closed down 13 percent at CNY22.76 (roughly USD3.27), marking the largest one-day drop on the mainland exchanges.
Following recommendations from the national health regulatory authority, the company decided to pull its registration request voluntarily after thorough deliberation. It plans to gather additional clinical data and submit a revised application once these supplementary case studies are completed.
The drug in question, Peginterferon Alfacon-2 Injection, is a long-acting interferon developed in-house. It initially received approval in 2018 for treating chronic hepatitis C in adults. However, with more hepatitis C therapies entering the market in recent years, the company shifted its focus toward developing treatments for hepatitis B.
In September 2024, the company submitted a new application to the regulatory agency to use Peginterferon Alfacon-2 in combination with Tenofovir Alafenamide Fumarate tablets for treating adult patients with chronic hepatitis B. Notably, this was the world’s first clinical attempt to combine a long-acting interferon with nucleoside analogs targeted at curing chronic hepatitis B.
Hepatitis B remains a major global health challenge. Left untreated, acute infections can develop into chronic hepatitis B, which may then lead to cirrhosis or liver cancer if not managed promptly. Currently, no drugs provide a complete cure for chronic hepatitis B; existing treatments only suppress the virus and slow disease progression.
The company disclosed it has set aside provisions for asset impairment related to the project’s development costs, which will result in a CNY111.3 million (about USD16 million) reduction in profit for 2025. An insider clarified that this move was a precautionary step, aligning with standard practices in the pharmaceutical industry, and does not imply a permanent halt to the project.
Founded in 2008, the company specializes in the research and development of innovative therapies for viral and immune-related diseases. Its financial results for the first three quarters of the previous year showed a 5 percent decline in net profit to CNY103 million (approximately USD14.8 million), with revenue dropping 8 percent to CNY927 million, according to its latest financial report.